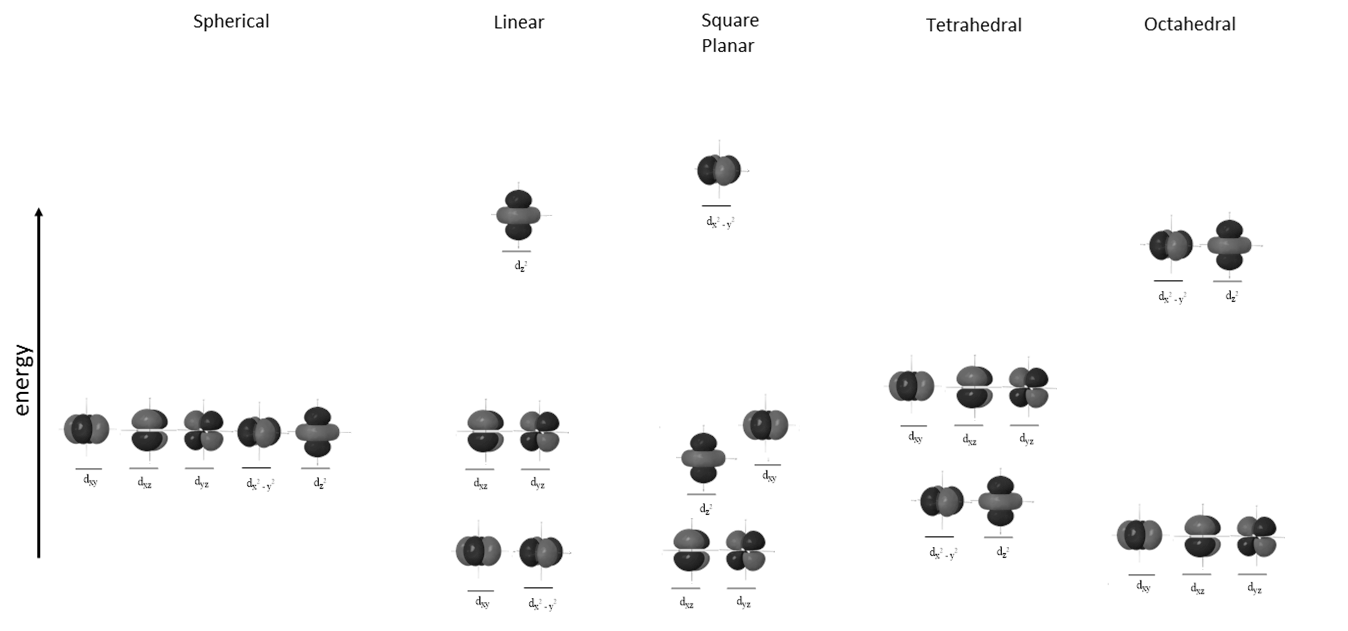
As we saw with the octahedral complex before, d-orbitals "split" into two different sets of orbitals differing in energy. In other arrangements of ligands around a metal center, d-orbitals also "split" into different sets based on energy:
 | |
|
6. How many ligands would be around the metal center in each of the above arrangements?
7. Based on this information, rank order the various geometries in order of likelihood of absorbing high-energy photons (ranging from lower-energy photons to higher-energy photons), assume V (II) ion with three electrons in the d orbitals. | |
| Back | Next |
|---|---|