|
Crystal Field Theory
According to Crystal Field Theory, when ligands are bound to the metal center, attractive and repulsive forces between the ligand and the d-orbitals of the metal cause the d-orbitals to change in energy. Take the octahedral example below. As six ligands move to bond with the metal, notice how the d-orbitals change in potential energy. Recall that the closer that like-charged particles are to one another, the more repulsion and greater potential energy: 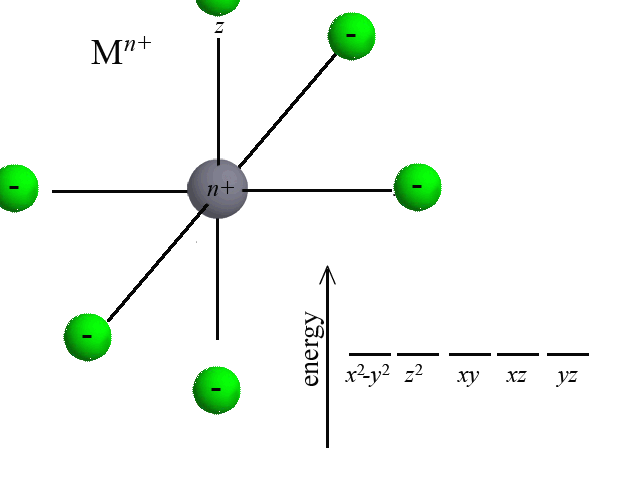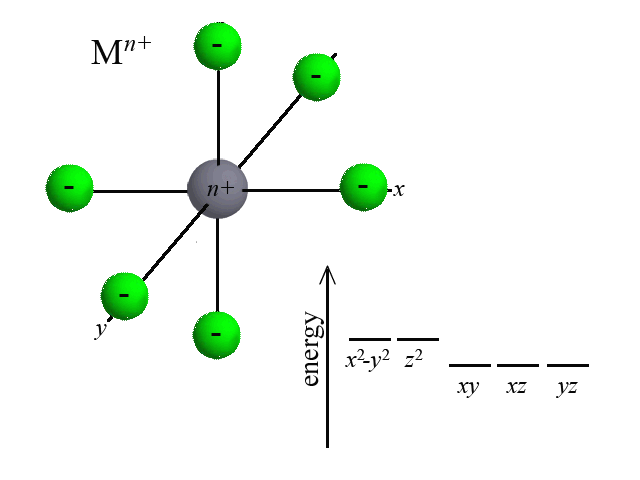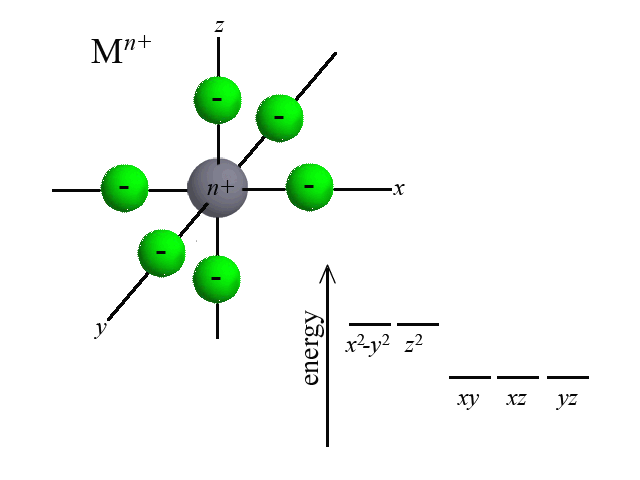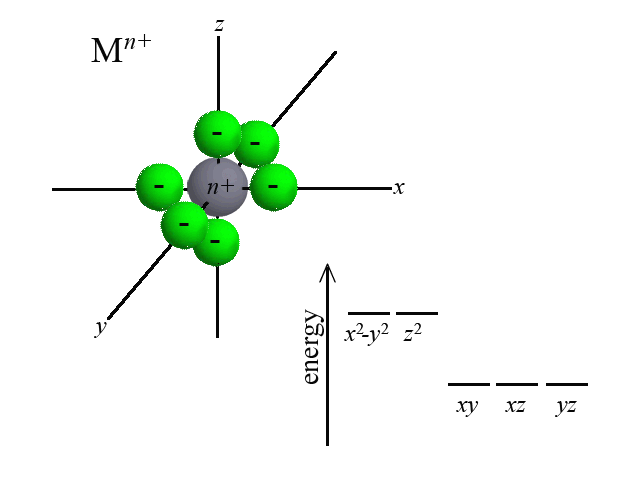 | |
| Back | Next |
|---|---|