
| Organic Chemistry Videos |

|
| Miami University |
 |
Standard Lab Write-up Laboratory Techniques Protocol for running Gaussian |
|
The research in my group currently are centered around two areas: (1) organic synthesis including total synthesis of natural products and the development of methodology; (2) study of nonbonding interaction involving aromatic rings. My group has accomplished the total synthesis of a dozen of different natural products. We have also made contributions in the understanding of the nonbonding interactions involving aromatic rings. Recently we have discovered a transannular [4+3] cycloaddition reaction. The transannular version of Diels-Alder reaction has been known for nearly two decades and has been shown to generate a high degree of stereochemical and architectural complexity in a single transformation. The newly discovered transannular [4+3] cycloaddition can match the efficiency of the corresponding Diels-Alder reaction, and produce multiple carbocycles with a seven-membered center ring, complementary to the transannular Diels-Alder reaction. The potent anti-angiogenesis natural products cortistatin A contains a central oxabicyclo[3.2.1] octane system, which can be efficiently constructed by the transannular [4+3] cycloaddition approach. 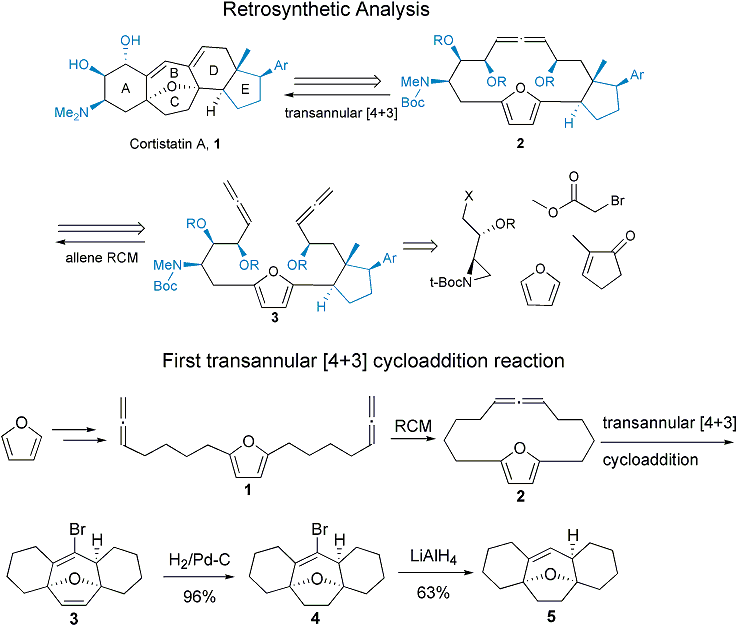
We have accomplished the total syntheses of several polyacetylenic and carbohydrate-containing natural products. Many of these polyacetylenes are isolated from the marine sponge, Petrosia sp. and have been identified as a unique class of natural products possessing diverse biological activities such as antimicrobial, antifungal, antifouling, H +, K+, -ATPase inhibitory, HIV inhibitory, immunosuppressive, and anti-tumor activities. 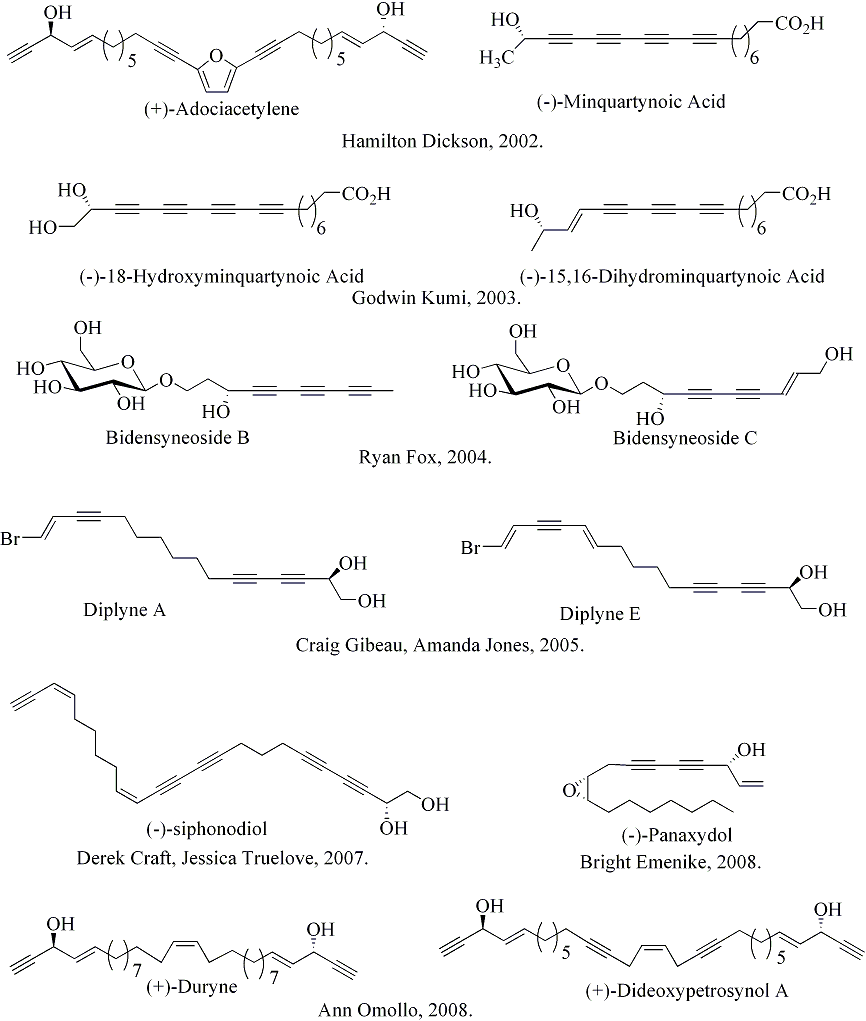
|
| Minquartynoic Acid (pdf)
|
| 18-Hydroxyminquartynoic Acid (pdf)
List of Publications |
| Main Page : Research : Group Gallery : Classes | |
|
|