|
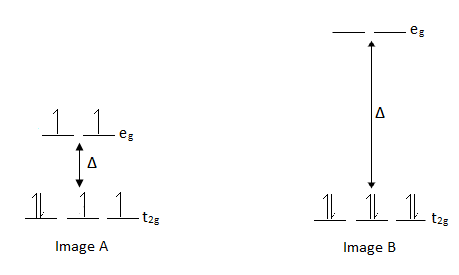
Therefore, the more π-accepting capability of a ligand, the larger the Δ; the more π-donating capability of a ligand, the smaller the Δ.
The way electrons occupy d-orbitals that are split is affected by the magnitude of the split/difference between the sets of orbitals (Δ). Image A represents the d-orbitals of a π-donating ligand/metal complex. Image B represents a π-accepting ligand/metal complex. |
|
 |
|
| 12. Describe, in your own words, how Δ affects how electrons occupy d-orbitals. | |
| Back | Next |
|---|---|