 |
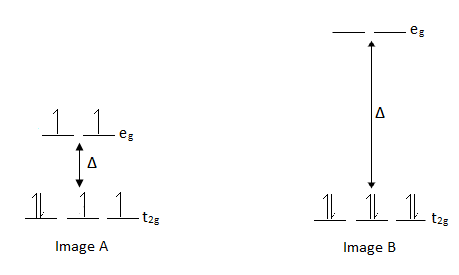
The electron configuration with the lowest potential energy is always favored. Recall Hund's rule which states that when orbitals are of equal energy, the electron configuration of lowest potential energy has the maximum number of unpaired electrons with parallel spins. However, in an octahedral arrangement, the d-orbitals split so all five orbitals are no longer equal in energy. |
|
13. Notice in Image B there are more electron pairs than in Image A, why?
14. Explain the differences in Image A and Image B in terms of the relative energies of the electrons. |
|
| Back | Next |
|---|---|